Our research revolves around the development of new chemical modification approaches for the creation of new forms of nucleosides and oligonucleotides of biological and therapeutic potential. Special attention is given to oligonucleotide-based tools (mainly stimuli-responsive) for the treatment of cancer and other multi-factorial disorders.
Stimuli-responsive oligonucleotide-based tools as promising candidates for cancer treatment
Oligonucleotide therapeutics are a very flexible platform for developing drugs for a wide range of diseases. However, their clinical application faces important hurdles, such as low biostability and poor cell uptake. Despite the significant clinical advances made in the last decade, there is still room for improvement in areas such as cancer and other important multifactorial disorders.
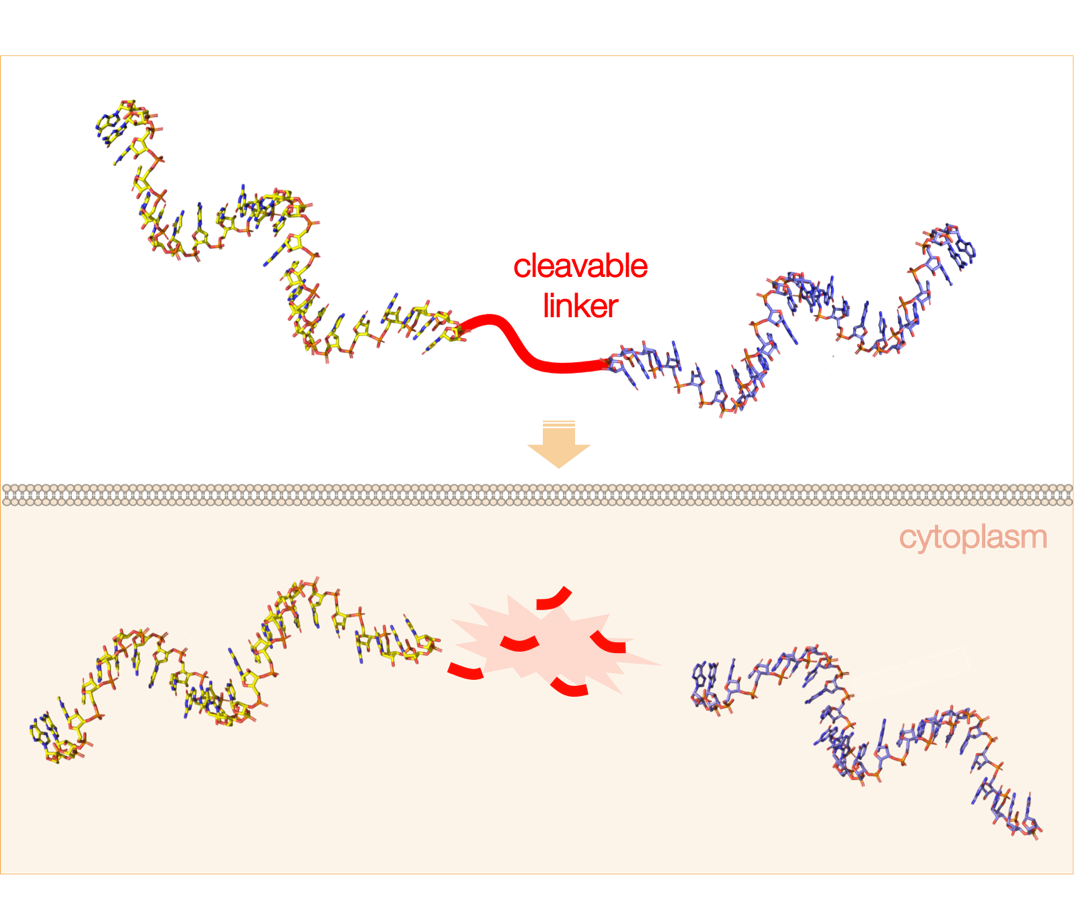
An important part of our research is focused on the development of modified oligonucleotide tools with the potential to tackle multifactorial disorders (among them, cancer and related drug resistance issues), while overcoming the limitations of oligonucleotide therapeutics. Among the different oligonucleotide tools that we are studying, special emphasis is placed on novel multi-target nanostructures specifically designed to administer several oligonucleotide drugs in a single dose (each of them active against a different therapeutic target) after their intracellular disassembly upon recognition by endogenous agents such as enzymes. In addition, their functionalization with accessory molecules, such as fluorophores and targeting peptides for monitoring their specific delivery to tumours.
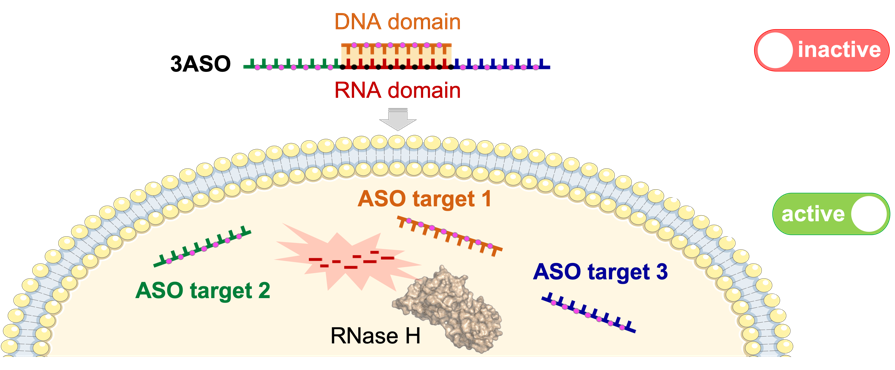
In this line, we have recently developed a new class of RNase H-sensitive construct (3ASO) that can be disassembled intracellularly after recognition and cleavage by endogenous RNase H enzyme, leading to the simultaneous release of three different therapeutic oligonucleotides (ONs), tackling each of them the mRNA of a different protein. As a model system for our technology we have created 3ASO constructs designed to specifically inhibit the expression of HER2, Akt and Hsp27 in HER2+ breast cancer cells. These trifunctional ON tools displayed very low toxicity and good levels of antiproliferative activity in HER2+ breast cancer cells. Additionally, this work has allowed us to study an unprecedented mode of RNase H recognition and function that is mainly dictated by the topology of our DNA·RNA-based construct.
Mata-Ventosa, A. Vila-Planas, A. Solsona-Pujol, J. de la Dueña, M. Torrents, E. Izquierdo-García, M. Pastor-Anglada, S. Pérez-Torras, M. Terrazas. Bioorg. Chem. 2024, 150, 107595 | DOI: 10.1016/j.bioorg.2024.107595
Extrapolation of our stimuli-responsive approaches to other classes of complex disorders
Our cleavable ON-based multi-target tools have the great potential to overcome the limitations typical of drug combinations of small molecules, while expanding the use of ON therapeutics to the treatment of multi-factorial complex disorders. For example, by simply changing the sequence of their ON components, the same scaffold can be used to inhibit the expression of virtually any combination of disease-related proteins. Another goal of our research is to extrapolate our stimuli-responsive oligonucleotide-based approaches to other classes of complex disorders (different from cancer), including viral infections, among others.
Oligonucleotide-based stimuli-responsive supramolecular materials of therapeutic potentail
Stimuli-responsive approaches such as the RNase H one have many other potential applications, not only in biomedicine but in nanotechnology. Another goal of our research is to apply our optimal stimuli-responsive oligonucleotide-based designs to develop new dissipative responsive supramolecular materials of therapeutic potential.